Definitions
Buoyancy – is the upward force that keeps things afloat. The net upward buoyancy force is equal to the magnitude of the weight of fluid displaced by the body. This force enables the object to float or at least seem lighter. Also known as Archimedes Principle is any object, wholly or partially immersed in a fluid, is buoyed up by a force equal to the weight of the fluid displaced by the object. Archimedes' principle does not consider the surface tension (capillarity) acting on the body. The weight of the displaced fluid is directly proportional to the volume of the displaced fluid (if the surrounding fluid is of uniform density). Thus, among completely submerged objects with equal masses, objects with greater volume have greater buoyancy.
Density – is defined as its mass (g) per unit volume (cm³) which is written as g/cm³. An easy definition for density is how much stuff (mass) is in how much space (volume).
Displacement – occurs when an object is immersed in a fluid (water) pushing it out of the way and taking its place. The volume of the fluid displaced can then be measured and from this the volume of the immersed object can be deduced (the volume of the immersed object will be exactly equal to the volume of the displaced fluid). Displacement can be used to measure the volume of a solid object of irregular form. Several methods of such measuring exist. In one case the increase of water level is registered as the object is immersed into the water. In the second case the object is immersed into a vessel full of water, causing it to overflow. Then the overflown water is collected and its volume measured.
Fluid – any liquid or gas.
Gravity or Gravitation - states that objects with mass attract one another or the pull they exert on each other. In everyday life, gravitation is most familiar as the agent that lends weight to objects with mass and causes them to fall to the ground when dropped. It causes dispersed matter to coalesce or come together as in star system formation.
Mass – in everyday usage is often taken to mean weight or the gravitational pull exerted on an object. In other words, how much stuff? However mass is a universal constant while weight is specific to a body's gravity.
Matter – is anything that has mass and occupies a volume; states of matter include solids, liquids, gases and plasma.
Specific Density – is an object’s density with respect to water (H₂0); which has a density of 1.0g/cm³. It is also known as relative density.
Viscosity - the thickness or thinness of a fluid. The fluid's resistance to flow.
Volume – is how much three-dimensional (3-D) space matter occupies and is commonly presented in units such as cubic centimeters (cm³) or milliliters (ml). *Please note that 1cm³ = 1ml, but always use cm³. In other words, how much space? While regular, straight-edged and circular shapes can be easily calculated using mathematic formulas, irregular shapes cannot. But the volume of any shape including both regular and irregular can be determined by displacement.
 |
||
|
Density is the mass of an object divided by its volume.
|
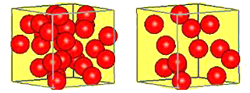
Take a look at the two boxes below. Each box has the same volume. If each ball has the same mass, which box would weigh more? Why?
 |
||
|
A box with more particles in it will be more dense than the same box with fewer particles.
|
The box that has more balls has more mass per unit of volume. This property of matter is called density. The density of a material helps to distinguish it from other materials. Since mass is usually expressed in grams and volume in cubic centimeters, density is expressed in grams/cubic centimeter.
How do I calculate density?
(Courtesy of the Science Education Research Center Carlton University)
Density often has units of grams per cubic centimeter (g/cm³). Remember, grams is a mass and cubic centimeters is a volume (the same volume as 1 milliliter). A box with more particles in it will be denser than the same box with fewer particles.
Density is a fundamental concept in the sciences; you will see it throughout your studies. It is used quite often in identifying rocks and minerals since the density of substances rarely changes significantly. For example, gold will always have a density of 19.3 g/cm³; if a mineral has a density other than that, it isn't gold.
You probably have an intuitive feeling for density in the materials you use often. For example, sponges are low in density; they have a low mass per unit volume. You are not surprised when a large sponge is easy to lift. In contrast, iron is dense. If you pick up an iron skillet, you expect it to be heavy. On the other hand, students, and even teachers, often confuse mass and density. The words heavy and light on their own refer to mass, and not density. A very large sponge may weigh a lot (have a high mass), but its density is low because it still weighs very little per unit of volume. For density, you also need to consider the size, or volume, of the object.
More Heat, Less Density
The density of a gases, liquids and even solids changes when its temperature changes. When air or water is heated, it expands, or takes up more space. As the air or water expands, its density decreases — hot air or water is less dense than cool air.
How do I determine density?
Density is not something that is directly measured. Typically if you want to know the density of something you will weigh it and then measure its volume.
 |
||
|
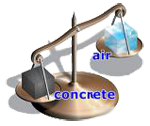
A concrete cube will weigh more than a cube of air the same size because it is denser.
|
Example - You collect a boulder and take it back to the lab, where you weigh it and find its mass to be 1000g. You then determine the volume is 400cm³. What is your boulder's density? Density is mass divided by volume, (d=m/v). In this case the mass is 1000g and the volume is 400cm³, so you divide 1000g by 400cm³ to get 2.5 g/cm³.
Another tricky thing about density is that you can't add densities. If I have a rock that is made up of two minerals, one with a density of 2.8 g/cm³, and one with a density of 3.5 g/cm³, the rock will have a density between 3.5 and 2.8 g/cm³, not a density of 6.3 g/cm³. This is because both the mass and the volume of the two minerals will be added, and so when they are divided to get the density the result will be between the two.
Typical densities for gasses are on the order of thousandths of grams per cubic centimeter. Liquids often have densities of about 1.0 g/cm³, and indeed, fresh water has a density of 1.0 g/cm³. Rocks often have a density around 3 g/cm³, and metals often have densities above 6 or 7 g/cm³.
How do I calculate specific gravity?
To calculate the specific gravity (SG) of an object, you compare the object's density to the density of water:
Because the density of water in g/cm³ is 1.0, the SG of an object is will be almost the same as its density in g/cm³. However, specific gravity is a unit-less number, and is the same in the metric system or any other measurement system. It is very useful when comparing the density of two objects. Since specific gravity is unit-less, it doesn't matter whether the density was measured in g/cm³ or in some other units (like lbs/ft³).
You have a sample of basalt with density 210 lbs/ft³. The density of water is 62.4 lbs/ft³. What is the specific gravity of the basalt?
The specific gravity is the density of the substance divided by the density of water, so... we divide the basalt (210 lbs/ft³) by the density of water (62.4 lbs/ft³), and get S.G.= 3.37.
Finding Mass with a Digital Scale